This complement C3-inhibitor has passed phase 2 clinical studies in terms of safety and efficacy and is on track to becoming the first drug approved for the treatment of geographic atrophy.
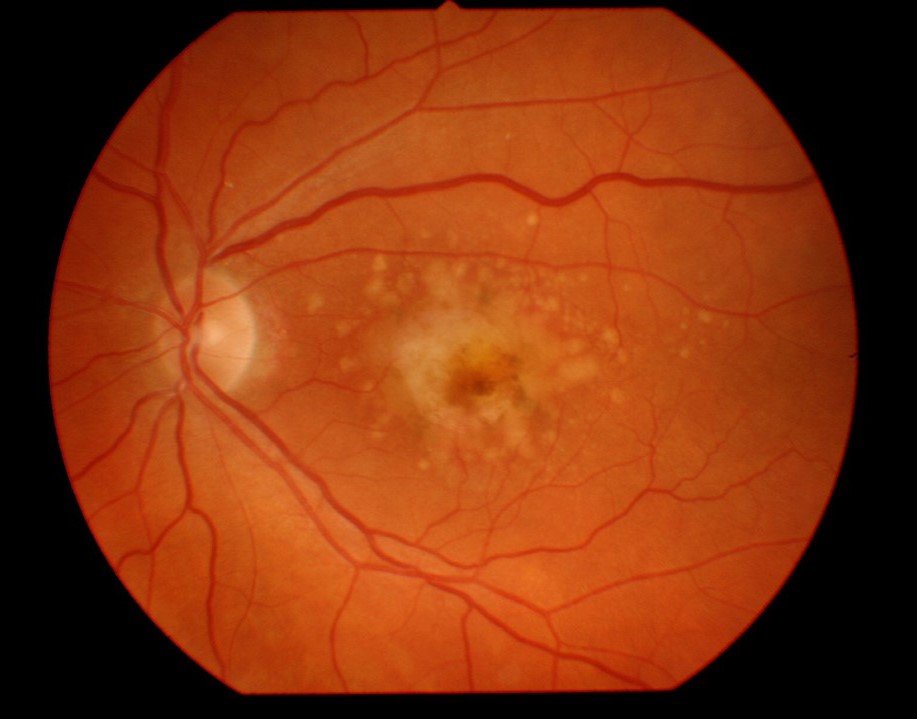
Fundus image of the left eye showing geographic atrophy, drusen and hemorrhage near the macula. Geographic atrophy is commonly associated with neovascular macular degeneration.
Geographic atrophy (GA) is a late stage of age-related macular degeneration (AMD), resulting in eventually irreversible vision loss and decreased quality of life. GA is also commonly associated with neovascular AMD, which can be treated with vascular endothelial growth factor (VEGF) inhibitors. However, there are presently no approved treatments for GA.
The dysregulation of the complement system has been shown to be a key factor in the development of AMD. Because of the multitude of biochemical molecules involved in several pathways, the complement C3 is deemed an attractive target because it sits at the juncture of complement pathways and is upstream of major effectors.
In this prospective, multi-center, randomized, sham-controlled phase 2 clinical trial, patients either received sham intravitreal injections or intravitreal injections of pegcetacoplan monthly or every other month (EOM) for 18 months. Pegcetacoplan is a complement C3 inhibitor peptide.
This paper’s findings are summarized in the following highlights:
- The GA lesion growth rate decreased by 29% and 20% in patients treated with pegcetacoplan monthly and EOM, respectively, when compared to sham treatment. This effect was more easily observed during the second 6 months of the study and was maintained thereafter, although the increased growth rate of the GA lesion after cessation of intravitreal injections suggested a need for continuous scheduled treatment.
- At month 12, pegcetacoplan had no effect on visual acuity measures and low-luminance visual acuity deficit, versus sham treatment.
- There was a higher incidence of exudative AMD in eyes treated with pegcetacoplan. However, this occurred mostly in patients with contralateral choroidal neovascularization (CNV). The incidence of CNV in the other eye of patients with exudative AMD is between 6-12%, as previously reported; the rates observed in this study were within this range.
This study demonstrated that C3 inhibition with pegcetacoplan can slow the progression of GA. Despite its limitations in outcome measures (e.g. the measurement of central vision, which can be unaffected until later in GA), the results have shown that the drug is of acceptable safety and efficacy for further studies in a phase 3 clinical trial.
Liao, D., Grossi, F., El Mehdi, D., Gerber, M., Brown, D., & Heier, J. et al. (2019). Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. doi: 10.1016/j.ophtha.2019.07.011